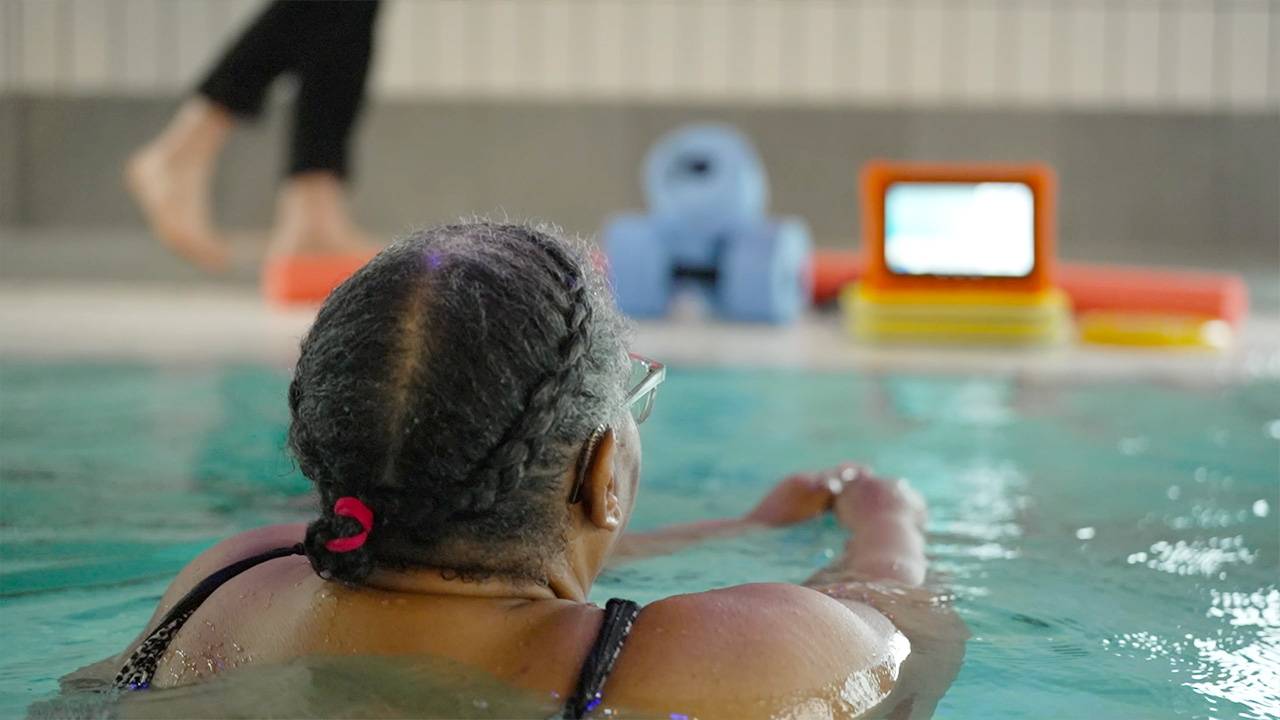Innovation
Transforming community places into therapeutic spaces for Musculoskeletal (MSK) conditions and wider long-term health condition self-management through a combination of technology and training
Project Lead
Ben Wilkins, CEO

About Good Boost
SBRI Healthcare supported Good Boost to develop the technology (both A.I. software and the rugged tablet-computer hardware) to enable the delivery of personalised programmes of therapeutic exercise and education in swimming pools and gyms.
The challenge was the accessibility of therapeutic exercise programmes for people living with MSK conditions that were suitable, safe and adaptable to an individual’s needs, ability and condition in a group class environment. The barriers for the delivery of these programmes included limited clinical skills and availability of clinical staff in community gyms and pools, and the cost of delivery of individually tailored programmes - often requiring a low ratio of participants to instructor.
SBRI Healthcare supported the development of group-based Good Boost programmes for people living with MSK conditions. The technology developed enabled mixed ability, mixed need, mixed condition participation, enabling classes of up to 20 participants to be active together. It also enabled large group classes to ensure the long-term sustainability of the programme due to their economic viability compared to 1-2-1 classes and classes of low ratio of participant to instructor.
Participants of Good Boost report a meaningful improvement in pain, physical function and quality of life, improving patient experience and health outcomes.
Impact
- E mark Class 1 Medical Device
- Selected for Microsoft’s ‘AI for Good’ accelerator and for the Institute for Ethical AI’s Catalyst programme, won the ‘Technology Award’ at the London Sports Awards
- Supported by Arthritis Action, the National Rheumatoid Arthritis Society, the National Axial
Spondyloarthropathy Society and Versus Arthritis - COVID-19: Awarded ‘Business-Led Response to COVID-19’ Innovate UK funding to develop a home app for land exercises to support people to manage their MSK conditions at home and maintain social distancing
- Awarded Pool Product of the Year’ 2020
- Delivered in over 200 venues since starting the SBRI Healthcare programme
- Working with 6 NHS Trusts to date, with many integrating their MSK pathway into Good Boost sessions in the community
- Good Boost is both NHS Digital Technology Certified (DTAC) and ORCHA medical app certified
- Evidenced to be effective for the improvement of pain, physical function, and overall quality of life
- Won 12 industry awards, including from the Royal Society of Public Health (RSPH) ‘Community Impact’ and ‘Public Health Minister Award’

"It’s made a heck of a difference. It’s taken me five years to find some exercise I can do. It came quite easy and it was enjoyable. I can now use my left leg to step up occasionally, and I could not do that before. I’m on my own, I can do something on my own!"
Nola patient
Dr. Nicky Wilson, Consultant Physiotherapist
"Some of the risks of not getting care include pain related disability, social isolation and psychological distress and a loss of work. Good Boost can be a channel for people to receive care because the wait for NHS services is often very long. Having a service like Good Boost that we can put into the community is a significant step towards reducing some of these health inequalities."

"I don’t know what I would do without Good Boost - it's great, I’ve been coming for quite some time. I went to the Physio and they told me the pain in my hip - they couldn’t do anything for me because it was due to the stroke. Good Boost is the best thing I could have done, it’s helped me so much with my balance and stopped making me feel isolated."
Good Boost Participant, Francis
Date published
December 2024